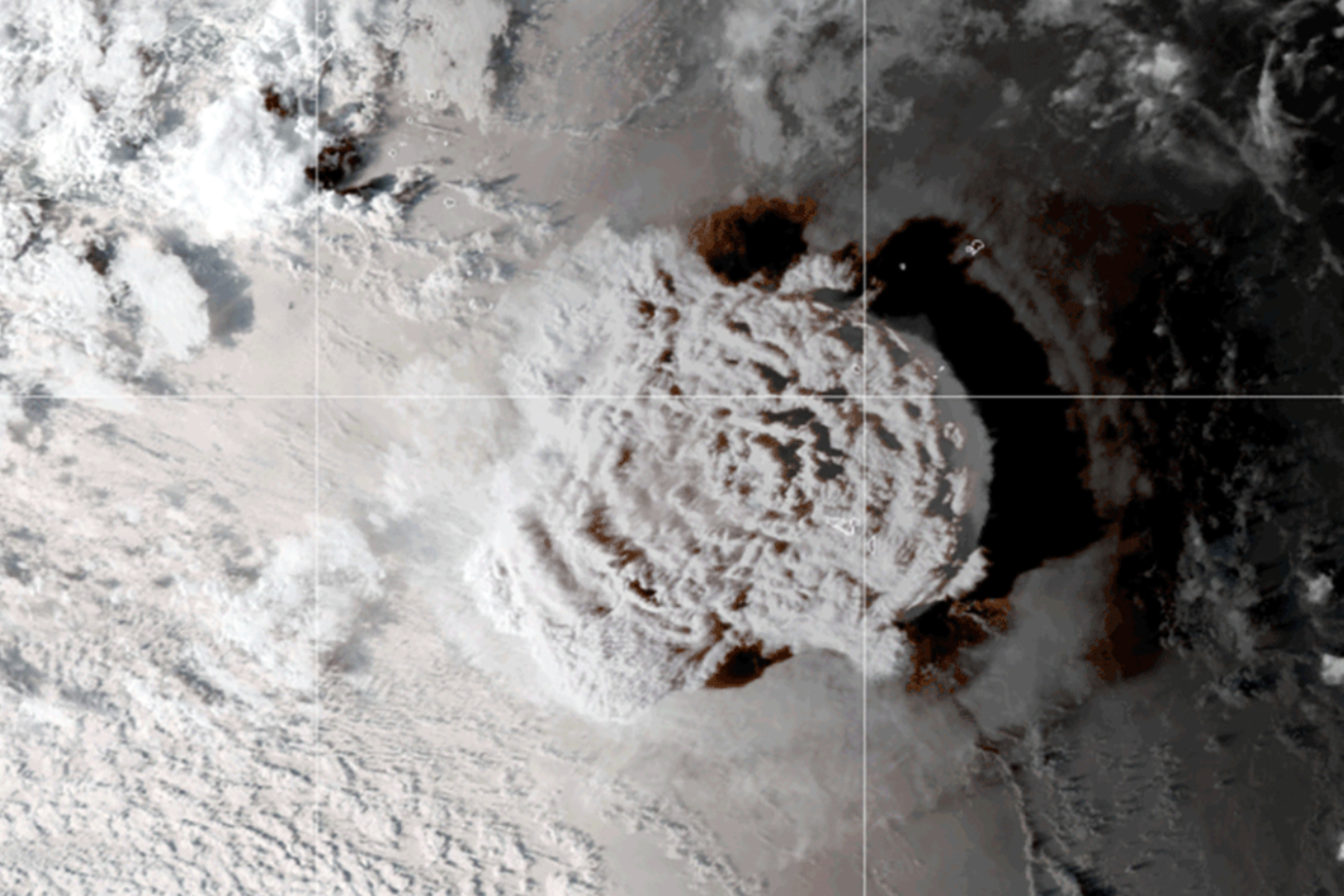
The 2022 eruption of the Hunga-Tonga Hunga-Haapai volcano tore a chunk out of the Earth’s ozone layer due to the huge volumes of water vapor it poured into the atmosphere, according to a new study published in the journal Science.
Situated on an island in Tonga, the volcano erupted on January 15 of that year, releasing 100,000 times more energy than the Hiroshima nuclear bomb in its huge explosion and matching Mount St. Helens’ power.
The eruption depleted the ozone layer by up to 5 percent in some regions within a single week of the event.
This was as a result of the enormous volumes of water pumped into the atmosphere from the eruption—alongside black ash, hydrochloric acid (HCl) and sulfur dioxide—forming plumes of vapor towering up to 34 miles high.
“During the Hunga Tonga eruption, a variety of substances were released into the upper atmosphere,” Stephanie Evan, lead author of the study and researcher at the Laboratoire de l’Atmosphère et des Cyclones (LACy), CNRS, Université de La Réunion, told Newsweek. “This included water vapor, sulfur dioxide (SO2), and volcanic ash. Specifically, SO2 is a prominent volcanic gas that can react with atmospheric water vapor to form volcanic aerosols, primarily composed of sulfuric acid. These aerosols have the capacity to scatter sunlight, impacting climate and playing a significant role in upper atmospheric chemistry, particularly with regard to ozone.”
This vapor reacted with a number of other chemicals shot out of the volcano, resulting in the breaking down of O3 ozone in the atmosphere above the tropical southwestern Pacific and Indian Ocean regions.
“Volcanic aerosols in the upper atmosphere play a significant role in ozone chemistry. These aerosols can facilitate chemical reactions that convert typically inactive gases into ozone-depleting molecules, notably chlorine atoms,” Evan explains.
“The increase in water vapor following the Hunga-Tonga eruption had a pivotal impact. It raised relative humidity and cooled the upper atmosphere, primarily between 25 and 30 km in altitude. This change in conditions allowed chemical reactions to occur on the surfaces of volcanic aerosols at temperatures higher than their usual range. The chemical reactions that occurred on the hydrated volcanic aerosols resulted in the creation of reactive chlorine compounds, like chlorine monoxide (ClO), from chlorine compounds that were not typically active, such as hydrogen chloride (HCl).”
The authors of the study described in the paper how they launched balloons from Réunion Island in the Indian Ocean into the volcanic plume, five days after the eruptions, in order to measure the chemical reactions occurring as it floated away into the atmosphere.
They found that the significant increase in water vapor and aerosol surface area was accompanied by substantial ozone depletion, at a rate of 0.07ppmv/day [parts per million volume per day]. They also reduced concentrations of HCl and increased ClO, indicating that the chlorine was reacting with O3 ozone, eroding the O3 levels in the atmosphere.
“In simple terms, the volcanic aerosols enabled the formation of substances that could break down ozone, contributing to its reduction. This transformation of chlorine species contributed to the fast reduction of ozone in the upper atmosphere above the tropical southwestern Pacific and Indian Ocean region in the week after the eruption,” Evan said.
Ozone concentrations in the region of the plume was found to have decreased rapidly by 5 percent in a single week. While huge, this is nowhere near the degree of reduction over the Antarctic, where the ozone hole depletes by around 60 percent between September and November each year. The disruption to the ozone over the tropics was unusual, as this area is usually very invariable in ozone thickness.
“One noteworthy aspect of the Hunga Tonga eruption was the injection of an unprecedented amount of water vapor to very high altitudes. During the campaign, we observed water vapor levels reaching at least 70 times the normal background levels,” Evan said. “This increase in water vapor was correlated with a decrease in ozone and the presence of aerosol layers. These aerosols facilitated chemical reactions that transformed typically inactive gases in the upper atmosphere into ozone-depleting molecules, such as chlorine atoms.”
The scientists expected that the volcanic plume would drift over the Antarctic, further thinning the hole over the southern pole of the planet. However, observations of the Antarctic ozone hole have shown that it remained unchanged by the volcano.
“As the volcanic plume from Hunga Tonga traveled over time within the tropics, it gradually dispersed. The predominant dispersion of the plume was towards the Southern Hemisphere midlatitudes, primarily due to the large-scale circulation patterns in the upper atmosphere,” Evan said. “During this dispersal, the concentration of water vapor in the plume decreased. Our research indicates that water vapor levels needed to be significantly elevated, at around 20 times the normal background levels, for the chemical reactions on volcanic aerosols that lead to ozone destruction. Consequently, as the plume dissipated, and water vapor levels returned to lower values by the end of January 2022, we observed a cessation of rapid ozone depletion in the NASA satellite measurements.”
The authors hope to use these findings to further study how natural disasters might impact the atmosphere, and therefore, climate change.
“The introduction of a significant amount of water vapor into the upper atmosphere, as observed during the Hunga-Tonga eruption, can have several implications for climate change. Water vapor is a potent greenhouse gas that absorbs heat in the form of infrared radiation from the Earth’s surface and subsequently re-emits it. Consequently, the injection of such a substantial quantity of water vapor is expected to contribute to warming in the atmosphere for a period of several years until the gas naturally dissipates,” Evan said.
“Moreover, the increased presence of water vapor may also have secondary effects on atmospheric chemistry, including processes that influence ozone levels. However, these specific implications were not within the scope of our study but are currently the subject of ongoing research within the scientific community.”
Do you have a science story to share with Newsweek? Do you have a question about volcanoes? Let us know via science@newsweek.com.
Update 10/20/23, 8:36 a.m. ET: This article has been updated with comment from Evan.